IMARC Group has recently released a new research study titled “Virtual Clinical Trials Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2024-2032”, offers a detailed analysis of the market drivers, segmentation, growth opportunities, trends, and competitive landscape to understand the current and future market scenarios.
The global virtual clinical trials market size is expected to exhibit a growth rate (CAGR) of 4.86% during 2024-2032. Increasing demand for patient-centric approaches, advancements in technology like telemedicine and wearables, the impact of the COVID-19 pandemic, the emphasis on real-world evidence, and collaboration among stakeholders and efforts to address disparities in trial participation are some of the key factors contributing to the market growth.
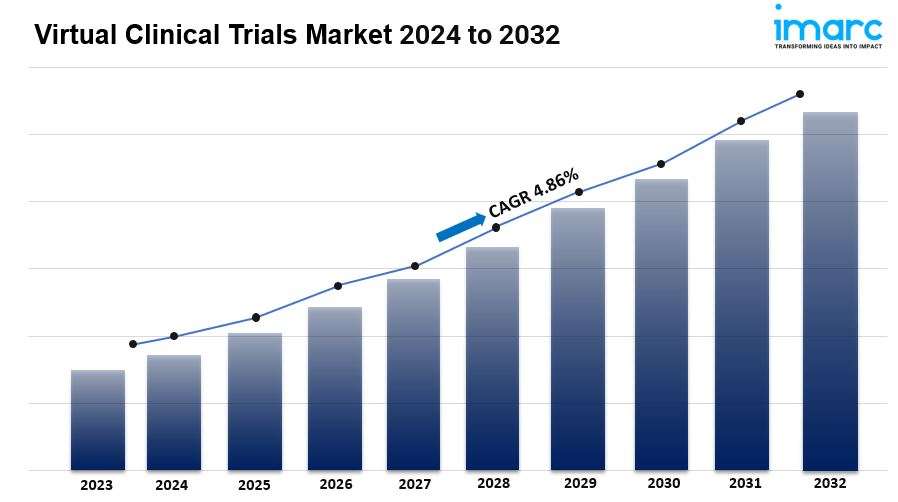
Global Virtual Clinical Trials Market Trends:
The heightened geographic diversity of participants through virtual clinical trials is boosting the market growth. Researchers can recruit participants from various regions and backgrounds, enhancing the generalizability of trial results and potentially uncovering variations in treatment efficacy across different populations. Additionally, the growing regulatory support and acceptance to support virtual clinical trials, recognizing their potential to enhance trial efficiency and patient safety, is fostering the market growth. Besides this, the rising focus on patient-centric approaches, where patient preferences and convenience are prioritized, leading to more patient-friendly trial designs, is contributing to the market growth. Furthermore, the rising prevalence of chronic diseases, necessitating more efficient and scalable clinical trial methods, is favouring the market growth.
Request to Get the Sample Report: https://www.imarcgroup.com/virtual-clinical-trials-market/requestsample
Factors Affecting the Growth of the Virtual Clinical Trials Industry:
Patient Convenience: Patient convenience is a critical factor driving the growth of the virtual clinical trials market. Traditional clinical trials often require participants to make frequent visits to clinical sites, which can be time-consuming and burdensome, especially for those with mobility issues, chronic conditions, or those living far from research centers. Virtual clinical trials address these challenges by allowing patients to participate from the comfort of their own homes. This flexibility reduces the need for travel and minimizes disruptions to participants’ daily lives, making it easier for them to adhere to trial protocols. Furthermore, the convenience of virtual trials can lead to higher enrollment rates, as more individuals are likely to participate when the process is less intrusive and more accommodating to their schedules. Additionally, virtual trials can provide more personalized and patient-centered care.
Cost Efficiency: Cost efficiency is a significant factor propelling the growth of the virtual clinical trials market. Traditional clinical trials are often expensive, with substantial costs associated with site maintenance, staffing, participant travel, and other logistical aspects. Virtual clinical trials offer a more cost-effective alternative by reducing or eliminating many of these expenses. For instance, the need for physical infrastructure, such as clinical sites and associated overheads, is greatly minimized. This reduction in physical site requirements translates to significant savings on rent, utilities, and maintenance. Additionally, virtual trials reduce the need for on-site staff, as many tasks can be managed remotely. This includes administrative roles, coordinators, and even certain medical professionals who can conduct virtual consultations. Furthermore, the use of digital tools and platforms for data collection, monitoring, and communication streamlines processes and reduces the time and resources required for data management and analysis.
Technological Advancements: Technological advancements are a cornerstone of the growth in the virtual clinical trials market. The development and integration of telemedicine and digital health technologies have revolutionized how clinical trials are conducted. Video conferencing tools have become more sophisticated, offering high-definition visuals and robust security features, enabling seamless and confidential interactions between researchers and participants. Remote monitoring devices, such as wearable sensors and mobile health applications, allow continuous and real-time data collection on various health metrics, such as heart rate, blood pressure, glucose levels, and physical activity. These devices can transmit data directly to researchers, reducing the need for frequent in-person visits and enabling more accurate and timely monitoring of participants’ health. Additionally, electronic data capture (EDC) systems have become more advanced, facilitating efficient and accurate data entry, storage, and analysis.
Explore Full Report Description At: https://www.imarcgroup.com/virtual-clinical-trials-market
Key Companies:
- Clinical Ink Inc.
- Covance Inc.
- ICON Plc
- IQVIA Inc.
- LEO Innovation Lab
- Medable Inc.
- Medidata Solutions Inc. (Dassault Systèmes SE)
- Medpace Holdings Inc.
- Oracle Corporation
- Parexel International Corporation (Pamplona Capital Management)
- PRA Health Sciences
- Signant Health (Genstar Capital)
Virtual Clinical Trials Market Report Segmentation:
By Study Design:
- Interventional
- Observational
- Expanded Access
Interventional accounted for the largest market share due to its structured approach in evaluating the efficacy of treatments under controlled conditions.
By Indication:
- Oncology
- Cardiovascular
- Others
Oncology represented the largest segment owing to the high prevalence of cancer and the significant investment in developing innovative cancer therapies.
Regional Insights:
- North America
- Asia-Pacific
- Europe
- Latin America
- Middle East and Africa
North America's dominance in the virtual clinical trials market is attributed to its advanced healthcare infrastructure, substantial funding for research, and supportive regulatory environment.
Speak to An Analyst: https://www.imarcgroup.com/request?type=report&id=3221&flag=C
Key Highlights of the Report:
- Market Performance (2018-2023)
- Market Outlook (2024-2032)
- Market Trends
- Market Drivers and Success Factors
- Impact of COVID-19
- Value Chain Analysis
- Comprehensive mapping of the competitive landscape
If you need specific information that is not currently within the scope of the report, we will provide it to you as a part of the customization.
About Us
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services.
IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: [email protected]
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145



